1,2-Amino Alcohols via Cr/Photoredox Dual-Catalyzed Addition of α-Amino Carbanion Equivalents to Carbonyls | Journal of the American Chemical Society
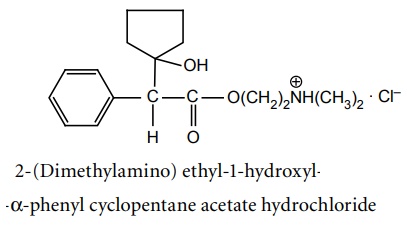
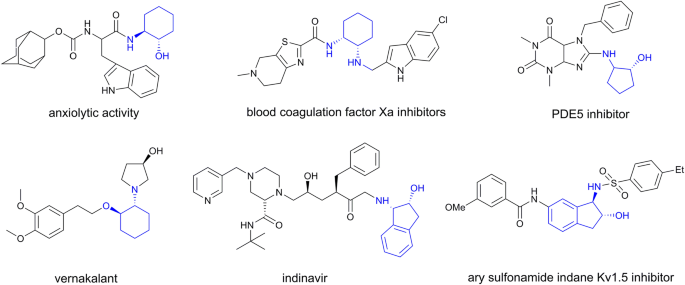
Amino alcohol esters - Structure, Properties, uses, Synthesis, Assay, Storage, Dosage forms, Dose | Synthesis and Drug Profile | Anticholinergic Drugs
![PDF] A Brief Review on Synthesis of ò-amino Alcohols by Ring Opening ofEpoxides | Semantic Scholar PDF] A Brief Review on Synthesis of ò-amino Alcohols by Ring Opening ofEpoxides | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/874e9622a53f180f841e605dba48a23615d350be/2-Figure3-1.png)
PDF] A Brief Review on Synthesis of ò-amino Alcohols by Ring Opening ofEpoxides | Semantic Scholar

1,2-Amino Alcohols via Cr/Photoredox Dual-Catalyzed Addition of α-Amino Carbanion Equivalents to Carbonyls | Journal of the American Chemical Society

Hydrogen‐Borrowing Alkylation of 1,2‐Amino Alcohols in the Synthesis of Enantioenriched γ‐Aminobutyric Acids - Hall - 2021 - Angewandte Chemie - Wiley Online Library

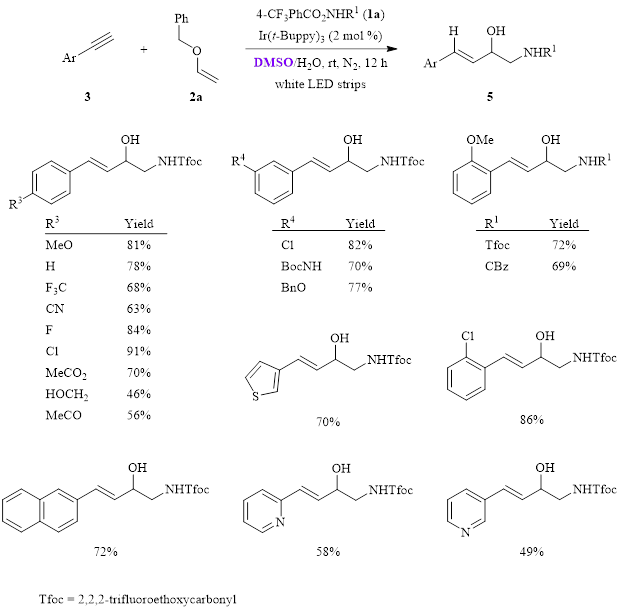
Synthesis of 1,3-Amino Alcohols, 1,3-Diols, Amines, and Carboxylic Acids from Terminal Alkynes | The Journal of Organic Chemistry
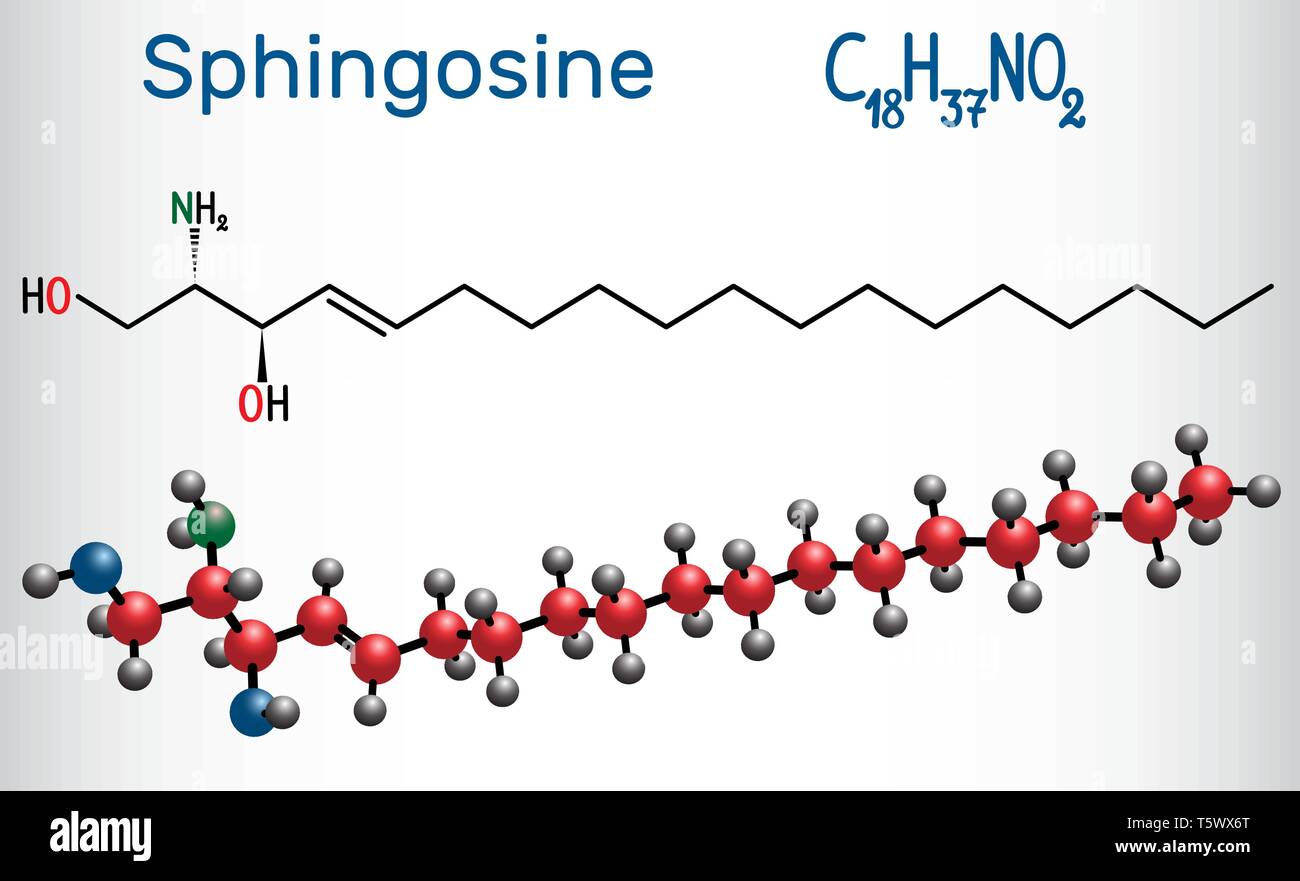
Sphingosine molecule . It is an amino alcohol , forms a primary part of sphingolipids. Structural chemical formula and molecule model. Vector illustr Stock Vector Image & Art - Alamy
High throughput solid-phase screening of bacteria with cyclic amino alcohol deamination activity for enantioselective synthesis of chiral cyclic β-amino alcohols | SpringerLink
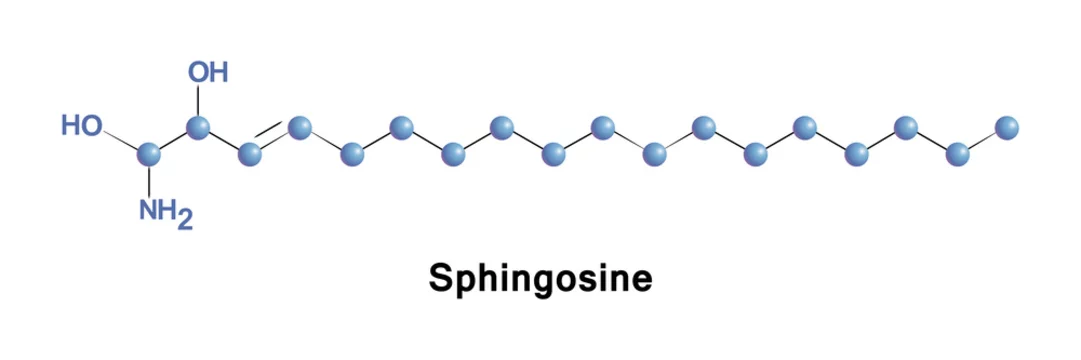
Sphingosine is an 18-carbon amino alcohol with an unsaturated hydrocarbon chain, forms a primary part of sphingolipids, a class of cell membrane lipids that include sphingomyelin, a phospholipid. Stock Vector | Adobe
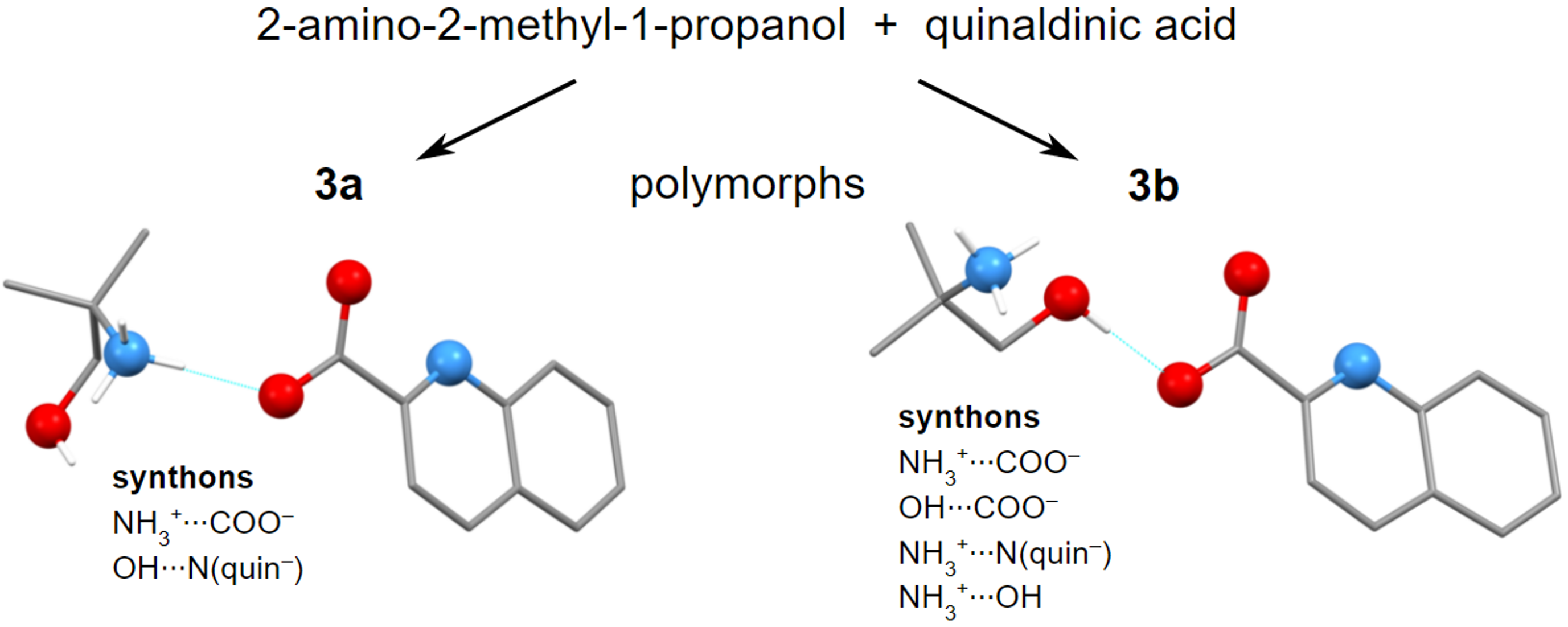
Molecules | Free Full-Text | Hydrogen Bonding and Polymorphism of Amino Alcohol Salts with Quinaldinate: Structural Study
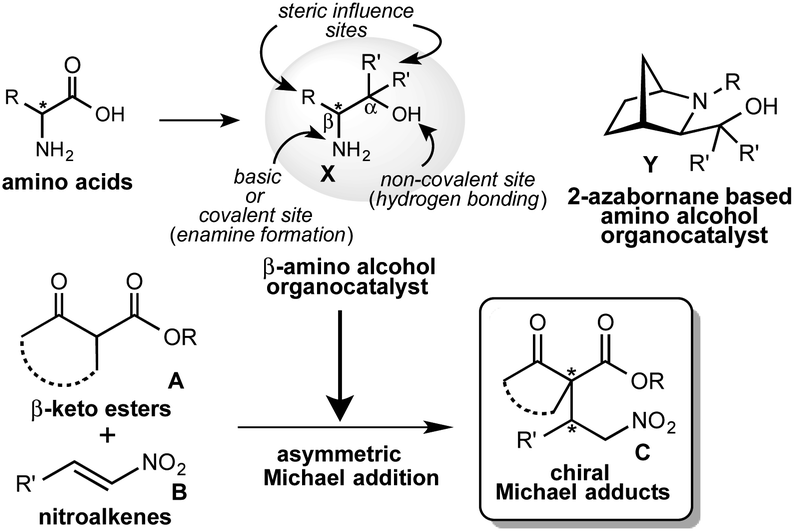
Simple primary β-amino alcohols as organocatalysts for the asymmetric Michael addition of β-keto esters to nitroalkenes - RSC Advances (RSC Publishing) DOI:10.1039/D0RA09041G
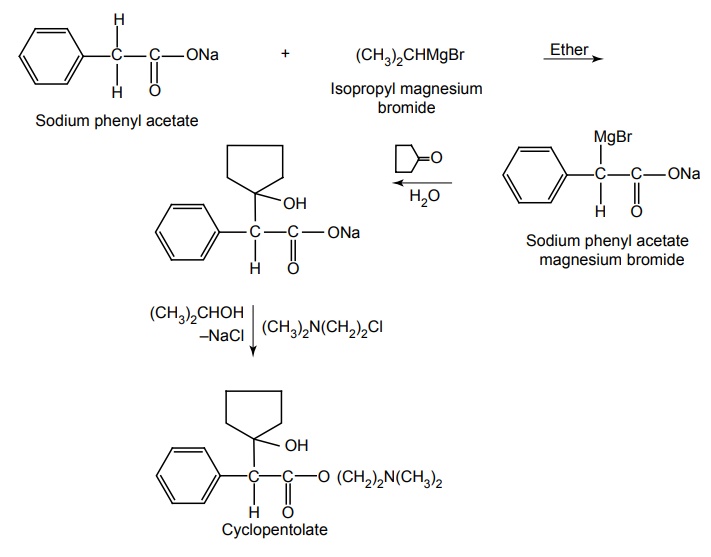
Amino alcohol esters - Structure, Properties, uses, Synthesis, Assay, Storage, Dosage forms, Dose | Synthesis and Drug Profile | Anticholinergic Drugs

Iridium-catalyzed enantioselective synthesis of chiral γ-amino alcohols and intermediates of (S)-duloxetine, (R)-fluoxetine, and (R)-atomoxetine | Communications Chemistry
![PDF] A Brief Review on Synthesis of ò-amino Alcohols by Ring Opening ofEpoxides | Semantic Scholar PDF] A Brief Review on Synthesis of ò-amino Alcohols by Ring Opening ofEpoxides | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/874e9622a53f180f841e605dba48a23615d350be/2-Figure1-1.png)
PDF] A Brief Review on Synthesis of ò-amino Alcohols by Ring Opening ofEpoxides | Semantic Scholar